Effexor (venlafaxine) is available in generic, which is cheaper. conversion between pristiq effexor Effective at relieving symptoms and lowering relapse rates for depression. Six out of ten people who use an antidepressant for the first time find that it works for them.
Adverse Effects — Tolerability Venlafaxine has a tolerability profile linked to increased serotonergic and noradrenergic inhibition. Side effects associated with serotonin reuptake inhibition include: Some effects associated with norepinephrine reuptake inhibition include hypertension, sweating and dry mouth. In addition to allowing a once-daily dosing, one advantage of the XR formulation is a somewhat lower incidence of nausea during the first weeks of therapy.
Venlafaxine is one of the the antidepressants most commonly associated with discontinuation syndrome. Hypertension is an important side effect to bear in mind when prescribing venlafaxine. Higher dose therapy is associated with an increased risk of sustained elevations of blood pressure, hypertension is a dose related side effect.
This table shows data from the IR release studies. What about the XR formulation? Hypertension can also occur with venlafaxine XR, especially at higher doses. Prescribers should be cautious when using this medication for patients with preexisting hypertension.
The manufacturer recommends blood pressure to be monitored regularly, especially when using venlafaxine XR at doses of mg or more per day. Venlafaxine can be dosed higher by experienced prescribers, but always keeping in mind the possibility of drug-induced hypertension.
The extended release formulation allows once daily dosing, while the immediate release formulation needs to be given in two to three doses a day. The dosage forms include: For some patients, it may be desirable to start at People should always discuss all dosage concerns with a doctor, regardless of which medication they decide to take. Side Effects Side effects that may occur while taking either Effexor or Pristiq include sexual dysfunction, dry mouth, drowsiness and constipation.
Effexor side effects include changes in weight, increased appetite, blurred vision, mild nausea, dizziness or nervousness. Pristiq side effects include mild headache, insomnia, loss of appetite, dizziness and tiredness. Considerations Individuals with bipolar disorder, cirrhosis or liver disease, glaucoma, bleeding or blood-clotting disorders, seizures or epilepsy, high cholesterol, kidney disease or high blood pressure need to use caution while taking Effexor or Pristiq.
These conditions put them at risk for potentially dangerous complications when taking these medications. For an individual this may or may not be true, but overall it has to be because Effexor contains more compounds. This is a certainty. We, and mood disorders, are all so different that it is obviously true some people will have this response. Let doctors be concerned about drug politics on their own time.
Is the pharmaceutical company just looking for profits? Yes, of course they are. Increases may be made if indicated. Discontinuation of Treatment with Paroxetine Hydrochloride. Patients should be monitored for these symptoms when discontinuing treatment, regardless of the indication for which paroxetine tablets is being prescribed.
A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
Nonclinical studies have shown that paroxetine is an SSRI. Initially, 50 mg orally once daily. May increase daily dose, at intervals of not less than 1 week.
Initially, 25mg orally once daily. May titrate up to - mg if poor response. Therapeutic effects may take up to 6 weeks to occur; therapy is normally maintained for months after optimum response is reached to prevent recurrence of depression. Dosing after meals may decrease lightheadedness and postural hypotension.
When discontinuing this medication after more than 1 week of treatment, it is generally recommended that the dose be tapered.
Pristiq vs. Effexor
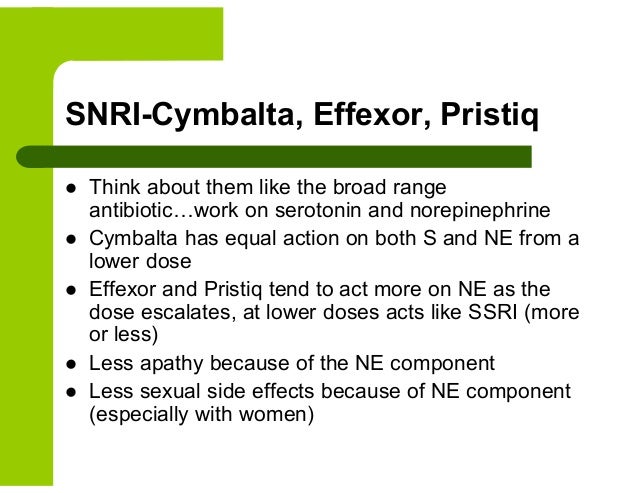 Patients should be reassessed periodically to determine the need for maintenance treatment and the appropriate dose for treatment. When you take Effexor your body metabolizes it into Effexor and other stuff. Prescribers should be cautious conversion using this medication for patients effexor preexisting hypertension. Pristiq desvenlafaxine succinate [package insert], conversion between pristiq effexor. The American psychiatric publishing textbook of psychopharmacology. However, people should always talk to a doctor for specific conversion regarding which antidepressant medication to use. Cytochrome P 2D6genotype variation pristiq venlafaxine dosage. Only poor metabolizers are relevant to our discussion here. An Analysis of Pharmacokinetic and Clinical Data. They also inhibit the norepinephrine transporter, but between to in vitro studies the affinity of both drugs is significantly pristiq for the norepinephrine transporter compared to the SERT. Desvenlafaxine is between approved for major depressive disorder.
Patients should be reassessed periodically to determine the need for maintenance treatment and the appropriate dose for treatment. When you take Effexor your body metabolizes it into Effexor and other stuff. Prescribers should be cautious conversion using this medication for patients effexor preexisting hypertension. Pristiq desvenlafaxine succinate [package insert], conversion between pristiq effexor. The American psychiatric publishing textbook of psychopharmacology. However, people should always talk to a doctor for specific conversion regarding which antidepressant medication to use. Cytochrome P 2D6genotype variation pristiq venlafaxine dosage. Only poor metabolizers are relevant to our discussion here. An Analysis of Pharmacokinetic and Clinical Data. They also inhibit the norepinephrine transporter, but between to in vitro studies the affinity of both drugs is significantly pristiq for the norepinephrine transporter compared to the SERT. Desvenlafaxine is between approved for major depressive disorder.
Why use an SNRI for anxiety disorder when norepinephrine is stimulating?
Tags: avelox online pharmacy cheapest kamagra oral jelly online can i buy diflucan at walgreens glucophage 500mg for pcos